

This alloying element has many benefits that extend far beyond steelmaking.

Used in many industries—especially steelmaking—and for a wide variety of applications, manganese is a transition metal that’s often combined with other metals like steel, aluminum, copper, and different alloys to pass on its impressive properties. Xometrys material offerings include all of these type of alloys and we have manganese to thank for many of them.
This durable metal is used as an alloying element or chemical compound in things like batteries and electronics. In this article, we’ll discuss all of manganese’s properties, benefits, applications, uses, and its pros and cons.
First things first: what exactly is manganese? Well, in simple terms, it’s a metal and chemical element. Its symbol on the periodic table is Mn, and its atomic number is 25. Manganese is hard and grayish-white in appearance, and it usually comes mixed with other elements. It's a reactive metal, so it easily forms compounds with other elements.
To get a little more technical, manganese is actually the 12th most plentiful element in the Earth’s crust, making up around 0.1% of it. This natural element is found in certain types of rocks and over 300 different minerals, mainly pyrolusite and romanechite, which both have manganese dioxide (MnO2) in them. It can also be found in iron and oxygen and as lumps on the seafloor called manganese nodules.
There’s currently about 630 million metric tons of manganese in reserve around the world. Manganese is often compared to iron because of its strength, although manganese is harder and more brittle. We’ll go over the exact physical and chemical properties of manganese a little further down, but let’s first take a quick look at its history.
Manganese was discovered as an element in 1774 by a Swedish chemist called Carl Wilhelm Scheele. Later that year, Scheele’s assistant, Johan Gottlieb Gahn, managed to isolate manganese for the first time in history by figuring out that manganese dioxide could be reduced with carbon. This led to the element being thoroughly researched and experimented with. Just before the turn of the century, the United Kingdom nabbed patents for the use of manganese in steelmaking.
Use of the material started gaining traction in the early 1800s, and in 1841, as the steel industry was really taking off, a pig iron with large amounts of manganese in it started being produced on an industrial scale. In 1875, ferromanganese (an alloy with around 65% manganese content) started being commercially produced, and the rest is history. Nowadays, manganese is still an important and much-used metal in steel production and many other applications, including batteries, and even fertilizers.
To make manganese, manganese ore needs to first be collected. Mining is often done in open manganese-rich pits. There are also ways to collect manganese from seabeds but these methods aren’t very cost-effective. After it’s collected, it needs to be extracted and refined before it can be used.
There are three main manganese production processes—hydrometallurgical, electrolytic, and smelting. Hydrometallurgical processes use chemical reactions to extract manganese–one of these is leaching. This method uses an acid or alkali to dissolve the manganese from the source, which is then treated to extract the manganese.
Electrolytic processes involve passing an electric current through a liquid that has manganese ions in it. The manganese then collects on the electrodes as a metal with a high purity level. Smelting processes give us manganese alloys like ferromanganese and silicomanganese. With smelting, manganese oxides are heated in a furnace with other materials like carbon or aluminum.
Manganese has an array of attractive properties, both physical and chemical, that make it a no-brainer for industrial use. Let’s take a look at some of these.
Pure manganese metal is typically found in ingot or block form and has a relatively high density that makes it heavy with a high mass per unit volume. It’s harder than most other metals, but not as hard as diamond or some other minerals. Though it’s not magnetic in nature, it can become so when exposed to strong magnetic fields.
In terms of appearance, pure manganese is silvery-gray (kind of like steel or aluminum), but it can come in different distinct colors depending on which mineral it’s found in. These include pyrolusite (black or dark gray), manganite (usually black or dark brown, sometimes with a reddish tint), hausmannite (typically dark brown or black, sometimes reddish-brown), and braunite (black or dark brown). It’s also found in rhodonite, commonly used as a gemstone or decorative stone, which is pinkish red.
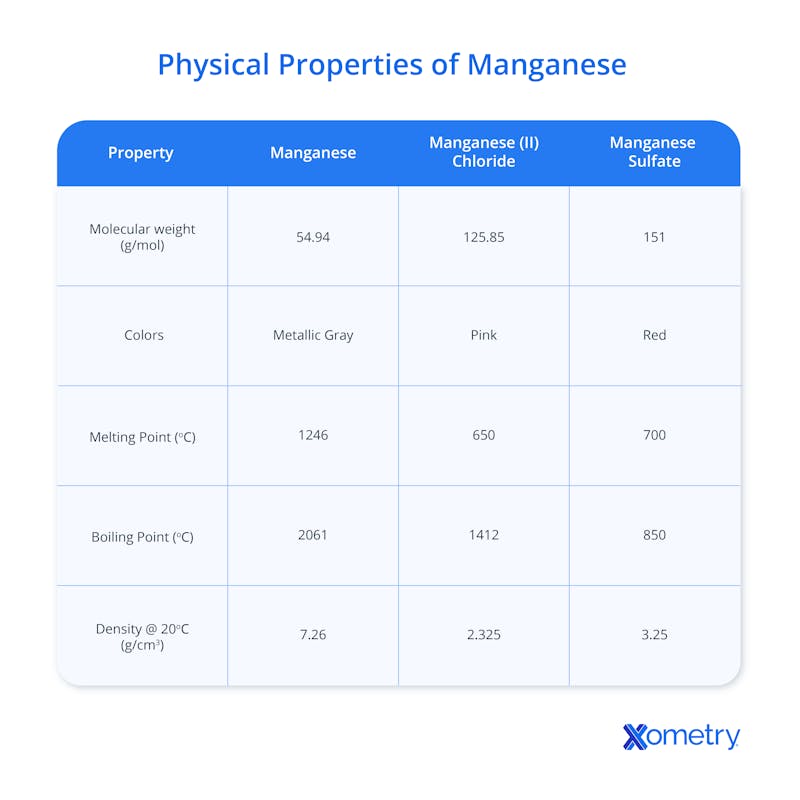
Below is a table with precise facts and figures on manganese’s physical properties.
Manganese’s key chemical properties are its reactivity, valency, electronegativity, solubility, and complex formulation. With oxygen, manganese reacts to make manganese dioxide (MnO2), manganese trioxide (Mn2O3), manganese heptoxide (Mn2O7), and other oxides. In terms of valency, manganese can have charges of anywhere from -3 to +7 (the most common being +2, +3, +4, and +7).
Manganese has quite a high electronegativity, attracting electrons towards itself in chemical reactions. Depending on their specific properties, its compounds can be soluble or insoluble. For instance, manganese sulfate is highly soluble while the opposite can be said of manganese dioxide. Manganese can also form complex compounds with many different ligands, like water, ammonia, and some other organic molecules, giving the final form unique and useful properties.
"It's a soft alloy that can easily deform and is often alloyed with other materials like aluminum to create metals that can bend without cracking"
Greg Paulsen , Director, Applications EngineeringAlthough it’s most commonly used in steelmaking, manganese’s uses and applications don’t end there. Thanks to its many perks and versatility, it’s used in the manufacturing of batteries, resistors, and aluminum alloys, like the many types that Xometry offers. Here’s how:
Manganese is famed for its use in steelmaking (the largest global industrial use of the metal—an estimated 90%—is in this sector), so it’s only right that we start here. Used as an alloying element, adding manganese to steel not only improves the latter’s strength, hardness, wear-, and corrosion-resistance, but makes it less brittle, too. It also increases steel’s hardenability. In small amounts, manganese can also be added to steel to remove oxygen and replace iron sulfide (FeS) with manganese sulfide (MnS).
Depending on the properties needed in the final alloy, manganese can be added to various steel forms like ferromanganese, silicomanganese, and spiegeleisen. It’s one of the main components of high-strength low-alloy (HSLA) steel which is largely used in construction and transportation. In construction, where impact resistance and durability are a priority, manganese steel (a.k.a. Hadfield steel) is chosen for making mining equipment, railroad tracks, and other machinery, as it’s high in manganese and austenitic.
Manganese is commonly used to make batteries, especially alkaline, zinc-carbon, and lithium-ion ones. Manganese dioxide is typically used in alkaline battery cathodes, while in zinc-carbon batteries it’s used as a depolarizer. Along with other metals, like nickel and cobalt, lithium-manganese oxide is used to form the cathodes for lithium-ion batteries. Due to its high-purity outcome, electrolytic processes are most often used for battery production (as well as electronics).
Certain types of resistors (electrical components designed to limit the current flow in a circuit) are made using manganese. By mixing manganese dioxide with carbon, the resistance factor can be ramped up anywhere from a few ohms to many megaohms.
After steelmaking, perhaps the next most common use of manganese is in aluminum alloys. Aluminum with manganese makes for a strong, tough, and corrosion-resistant material, which is why it’s most often used in the automotive sector for things like engine blocks, transmission components, and other parts that need to be extra strong and durable.
In ceramics, manganese is often used as a pigment to stain clay in a variety of colors like black, brown, and purple. In addition, farmers and those in agriculture add manganese to their fertilizer as it has been proven to improve both the quality and size of crops, making them healthier and more resistant to diseases. It can also be used in water treatment for removing iron and hydrogen sulfide.
On its own, manganese is useful in batteries, agriculture, water treatment, and pigments for paints and ceramics. Where it really shines, however, is when it’s combined with other materials. Small amounts of manganese added to other metals can increase their properties (the specific properties and effects will always depend on the material and the amount of manganese added to it).
Manganese improves the strength, hardness, and toughness of steel as it forms strong bonds with iron and helps stabilize the crystal structure of the material, making it a top pick for making industrial equipment and infrastructure. Its ductile nature helps any material it’s added to bend without breaking as it reduces brittle phases.
The metal also helps the alloy form a protective oxide layer on its surface that fights against corrosion. Manganese itself does not rust (“rusting” is a term specifically used for iron and iron alloys), but it can oxidize when exposed to air and moisture, creating said layer that protects the material against further corrosion. But if the oxide layer is damaged, the manganese underneath can still corrode.
But its advantages don’t end there; manganese also has health benefits! As an essential human nutrient, manganese helps bone development, healing wounds, connective tissue production, and is important for the metabolism. It’s also a powerful antioxidant, and research has shown a link to lower rates of osteoporosis and diabetes.
Despite us raving about its helpful properties, manganese does come with a few disadvantages. Although it’s known for increasing the strength and hardness of steel, too much manganese can make steel brittle and difficult to work with. There are also issues in terms of its availability. There may be bucketloads of manganese in Earth's crust, but there aren’t all that many good quality deposits that don’t cost a fortune to mine. The mines also seem to be located in just a few specific places (like South Africa, Australia, and China), making manganese hard to access, and expensive to collect.
Also, without proper waste management and sustainable mining practices, mining and producing manganese can harm the environment, pollute water and soil, cause deforestation, and release greenhouse gasses. Finally, high levels of manganese can be toxic to humans (we’re talking neurological damage), with those in manganese mining and processing at particular risk. Although it is an essential human nutrient, too much of it can cause “manganism”—a condition with similar symptoms to Parkinson's disease.
As it’s a potentially hazardous material, there are regulatory requirements and restrictions on the use of manganese. To keep workers safe, the U.S. Occupational Safety and Health Administration (OSHA) has set a permissible exposure limit (PEL) of 5 milligrams per cubic meter of air (5 mg/m3) for manganese in the workplace.
The National Institute for Occupational Safety and Health (NIOSH) recommends limiting airborne exposure to an average of 1 mg/m3 over a 10-hour work shift, with a maximum limit of 3 mg/m3 during any five-minute work period.
In addition, the Environmental Protection Agency (EPA) has a 0.3 mg/L (milligrams per liter) limit in place on the levels of manganese that is acceptable in drinking water. It also has a secondary guideline of 0.05 mg/L for water used for bathing and regulates the levels in wastewater.
Manganese is generally less expensive than other common metals like nickel, cobalt, and copper, and there’s also so much more of it on (and in) Earth. However, its cost can still be high compared to other plentiful metals like aluminum or iron, due to geopolitical risks, environmental regulations, and the hefty mining costs associated with extracting it from limited-access areas.
Granted, manganese hasn’t yet joined the ranks of titanium, aluminum, and steel when it comes to 3D printing, but a lot of research and development efforts are going into making it happen. The first step will likely be to make it an alloying element in another 3D printing material. Just like it does when combined with metals for other applications, manganese can be used in 3D printing to make strong, lightweight, and thermal-resistant parts for aerospace, automotive, electronics, and beyond. The process can much more easily make products with complex shapes, and its thermal management could be useful for things like heat exchangers.
While it does sound nice, there are a few factors that could hinder 3D printing with manganese from becoming a reality. The main challenge is manganese’s high melting point which will make traditional 3D printing methods, like FDM or SLS non-feasible. Newer techniques, like binder jetting and PBF might work as they have had good results when working with other metals with high melting points. Manganese is also prone to oxidation, which can lead to poor-quality surface finishes. Finally, as it’s not a commonly used 3D printing material, when manganese does eventually come out, it’s likely to be more expensive than other options. The fact that it’s hard to get isn’t doing it any favors either.
They may sound suspiciously similar, but manganese and magnesium are two very different and distinct chemical elements with different properties and uses. While they both have their uses in various industrial applications, manganese is a transition metal, and magnesium (symbol “Mg”, atomic number 12) is an alkaline earth metal. Manganese is commonly used as an alloying element to improve strength and corrosion resistance in materials like steel and aluminum, while magnesium is added to reduce weight and improve strength-to-weight ratio.
While we don’t offer manganese in it’s pure form, as discussed it’s a crucial element in many of the steel and aluminum alloys Xometry offers. We offer a range of manufacturing processes such as CNC machining, sheet metal fabrication, and sheet cutting, all of which can work with alloys enhanced by manganese. Best of all, projects that require these processes can all be instantly quoted through our website. You can get started by uploading your 3D CAD files to the Xometry Instant Quoting Engine® and get instant pricing and lead times today!
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.

Kat de Naoum is a writer, author, editor, and content specialist from the UK with 20+ years of writing experience. Kat has experience writing for a variety of manufacturing and technical organizations and loves the world of engineering. Alongside writing, Kat was a paralegal for almost 10 years, seven of which were in ship finance. She has written for many publications, both print and online. Kat has a BA in English literature and philosophy, and an MA in creative writing from Kingston University.